Describe Thomson's Concept of the Atom.
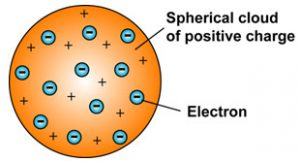
I An atom consists of a positively charged sphere and the electrons are embedded in it. Stoney had proposed that atoms of electricity be called electrons in 1894 surrounded by a soup of positive charge to balance the electrons negative charges like negatively charged plums surrounded by positively charged pudding.

Atom Discovery Of Electrons Britannica
Thomsons discovery of the electron completely changed the way people viewed atoms.

. Describe Thomsons concept of the atom. The number of protons equals the number of electrons in a neutral atom. Pages 9 This preview shows page 5 - 9 out of 9 pages.
2The positive and negative charges in an atom are equal in magnitudedue to which an atom is. In Thomsons model the atom is composed of electrons which Thomson still called corpuscles though G. 1An atom consist of a sphere of positive charge with negatively charged electrons embedded in it.
3 Drawdescribe Thomsons model of the atom In Thomsons model the atom is composed. In 1903 Thomson proposed a model of the atom consisting of positive and negative charges present in equal amounts so that an atom would be electrically. So the atom as a whole is electrically neutral.
Thomsons concept of the atom was referred to as the plum pudding model because it was randomly distributed electrons throughout an atom. The nucleus is a very small fraction of the total volume of the atom. Which led to plum pudding model.
Describe thomsons concept of the atom. Thomson was the first to put forward a model to explain the structure of an atom. An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude Thomson atomic model is compared to watermelon.
The goal of each atomic model was to present all the experimental evidence of atoms in the simplest way possible. In which he describe the resin as electrons and spherical body as central body in which electrons. Though several alternative models were advanced in the 1900s by Kelvin and others Thomson.
The electron was discovered by JJ. Thomsons atomic model William Thomson also known as Lord Kelvin imagined the atom as a sphere with a positive charge evenly distributed and embedded in it enough electrons to neutralize the positive charge. Thomsons atomic model is also called water melon model or Christmas pudding model.
Precisely speaking an atom consists of three major subatomic particles which are protons neutrons and electrons. Up until the end of the 19th century atoms were thought to be tiny solid spheres. This model did not tell us about the presence of neutrons in the atom.
3 drawdescribe thomsons model of the atom in thomsons. Thomson had discovered that atoms are composite objects made of pieces with positive and negative charge and that the negatively charged electrons within the atom were very small compared to the entire atom. Ii The negative and positive charges are equal in magnitude.
Jj thomson proposed his model of atom in the year 1903according to him-An atom consists of a sphere of positive charge with negatively charged electrons embeded in itThe positive and negative. Thomson Atomic Theory. Describe Thomsons concept of the atom.
Physics 21062019 1930 314180. The positive and negative charge is equal in magnitude and therefore an atom has no charge as a whole and is electrically neutral. Prior to Thomsons discovery the atom was thought to be indivisible.
According to Thomsons model the atom is like a plum pudding structurally. Thomson in the late 19th century. Thomson proposed the atom should be composed of electrons surrounded by a broth of positive charge to counteract the negative charges of the electrons.
According to the postulates of Thomsons atomic model an atom resembles a sphere of positive charge with electrons negatively charged particles present inside the sphere. Postulates of Thomsons atomic model Postulate 1. Thomson proposed his model of the atom in 1903then only electrons and protons were known to be present in the atom.
He compared the electrons with the raisins in the spherical Christmas pudding and to seeds in a watermelon. An atom consists of a positively charged sphere with electrons embedded in it Postulate 2. Thomson atomic model earliest theoretical description of the inner structure of atoms proposed about 1900 by William Thomson Lord Kelvin and strongly supported by Sir Joseph John Thomson who had discovered 1897 the electron a negatively charged part of every atom.
The Plum Pudding Model is a model of atomic structure proposed by JJ. In Thomsons model the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons negative charges like negatively charged plums surrounded by positively charged pudding. He believed that the electrons were like dry fruits in a.
Thomson Model of an atom. Course Title CHEM INORGANIC. The electrons make up the nucleus of the atom.
School Pickens County High School. The basic structure of an atom is defined as the component-level of atomic structure of an atom. The existence of protons was also known as was the fact that atoms were neutral in charge.
Since the intact atom had no net charge and the electron and proton had opposite charges the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. 2 Get Other questions on the subject. The mass of the atom is mostly the mass of the neutrons and protons.

Thomson Model Of The Atom Ppt Video Online Download
J J Thomson Model Of An Atom Class 9 Structure Of An Atom

Explain The Thomson S Model Of An Atom With A Neat Diagram Brainly In
Comments
Post a Comment